
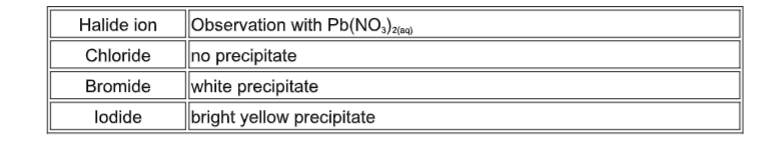

In addition to the bonding pair of electrons between the two atoms, each atom has 3 lone pairs of electrons in the outer shell. There must be another factor for consideration. The question is whether experimental data matches this prediction.Īs is clear from the figure above, the bond enthalpies of the Cl-Cl, Br-Br and I-I bonds decreases as predicted, but the F-F bond enthalpy deviates.īecause fluorine atoms are so small, a strong bond is expected-in fact, it is remarkably weak. As the atoms get larger down the group, the bonding pair is further from the nuclei and the strength of the bond should, in theory, decrease, as indicated in the figure below. In all halogens, the bonding pair experiences a net +7 charge from either end of the bond, because the charge on the nucleus is offset by the inner electrons. The figure below illustrates such a covalent bond: The extent of the attraction depends in part on the distances between the bonding pair and the two nuclei. It is that attraction which holds the molecule together. In both cases, about 99.5% of the halogen remains unreacted.Ĭovalent bonding is effective because the bonding pair is attracted to both the nuclei at either side of it. Bromine and iodine form similar compounds, but to a lesser extent. \Ĭhloric(I) acid is sometimes symbolized as HOCl, indicating the actual bonding pattern. The reaction is reversible, and at any time only about a third of the chlorine molecules have reacted. Chlorine reacts with water to some extent, producing a mixture of hydrochloric acid and chloric(I) acid (also known as hypochlorous acid). Iodine solution in water is very pale brown.
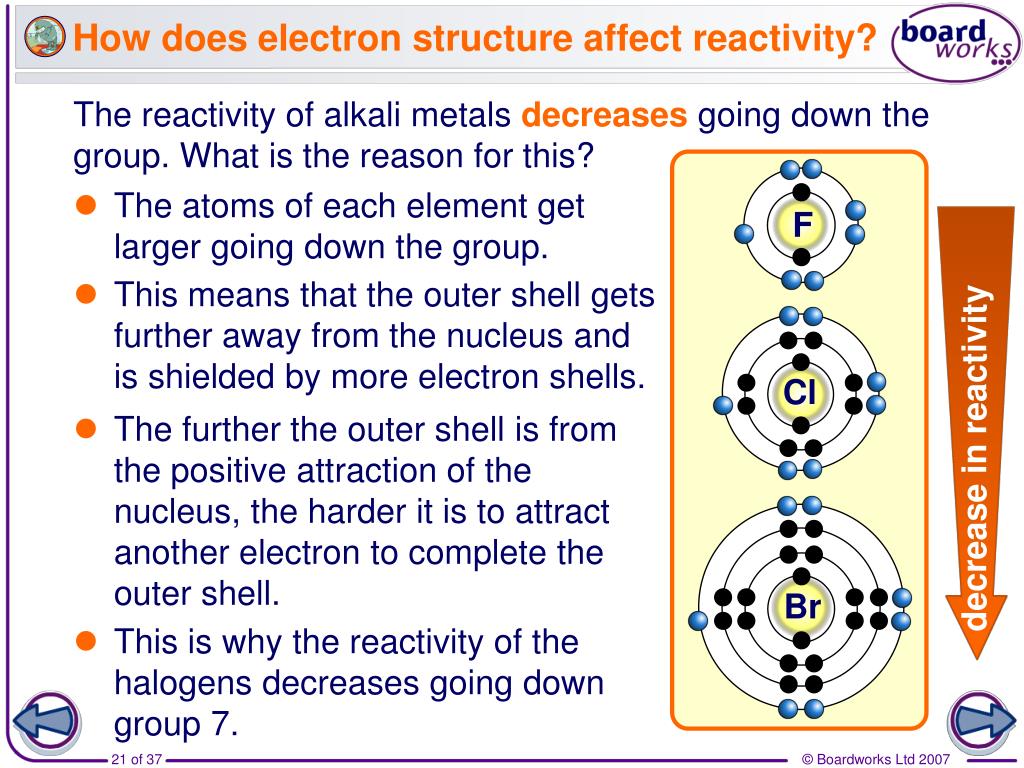
Bromine solution adopts a range of colors from yellow to dark orange-red depending on the concentration. The following table shows the solubility of the three elements in water at 25☌:Ĭhlorine dissolved in water produces a pale green solution. Chlorine, bromine, and iodine all dissolve in water to some extent, but there is again no discernible pattern. Therefore, the extra repulsion is particularly great and diminishes the attraction from the nucleus enough to lower the electron affinity below that of chlorine.įluorine reacts violently with water to produce aqueous or gaseous hydrogen fluoride and a mixture of oxygen and ozone its solubility is meaningless. The resulting repulsion from these electrons offsets some of the attraction from the nucleus.īecause the fluorine atom is very small, its existing electron density is very high. As the new electron comes approaches the atom, it enters a region of space already very negatively charged because of the existing electrons. However fluorine is a very small atom, with the incoming electron relatively close to the nucleus, and yet the electron affinity is smaller than expected.Īnother effect must be considered in the case of fluorine. The electron affinity therefore decreases down the group. In the larger atom, the attraction from the more positive nucleus is offset by the additional screening electrons, so each incoming electron feels the effect of a net +7 charge from the center.Īs the atom increases in size, the incoming electron is farther from the nucleus and so feels less attraction. The electron affinities generally decrease (meaning less heat is emitted), but the fluorine value deviates from this trend. Notice that the trend down the group is inconsistent. The trend down the group is illustrated below: There is a positive correlation between attraction and electron affinity. The electron affinity is a measure of the attraction between the incoming electron and the nucleus. The first electron affinities of the Group 7 elements


 0 kommentar(er)
0 kommentar(er)
